European Commission Announces Transatlantic Partnership with US EPA

[page_title] On 6 October, the European Commission announced the establishment of a transatlantic partnership between the Joint Research Centre and the US Environmental Protection Agency to exchange research materials and results useful for the development of integrated methods for predicting chemical toxicity.
October 2010 updates from carcinoGENOMICS, ESNATS and Sens-it-iv

[page_title] ecopa is dissemination partner in the EU 6th and 7th Framework Programme projects carcinoGENOMICS, ESNATS and Sens-it-iv. Brief updates from these projects are provided below, and comprehensive 2010 progress reports are available at AXLR8.eu. The carcinoGENOMICS project board met in Copenhagen, Denmark on 9-10 September to identify next steps to be completed leading up […]
EPAA Workshop Calls for Changes to Acute Toxicity Testing Requirements
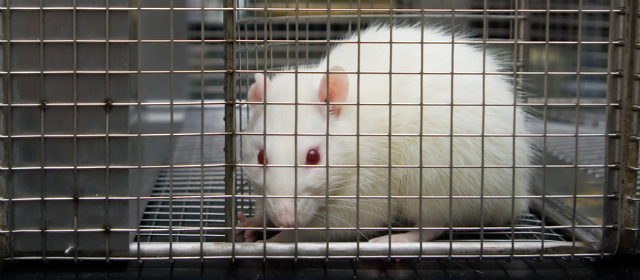
[page_title] On 16 September, the European Partnership for Alternative Approaches to Animal Testing (EPAA) convened a cross-sector workshop in Brussels to examine the findings of its task force regarding opportunities for application of the 3Rs in the area of acute systemic toxicity testing. Workshop participants included chemical and pesticide regulators from across EU member states […]
Europe Adopts New Law on Animal Experiments

[page_title] On 8 September, the European Parliament voted to adopt a new law replacing the nearly 25-year-old Directive 86/609/EEC, which regulates the use of more than 12 million animals in European laboratories each year. The new Directive 2010/63/EU introduces a number of substantial improvements, including: Mandatory authorisation and project evaluation for all applications to use […]
In Vitro Skin Irritation, Other 3Rs Guidelines Adopted by OECD
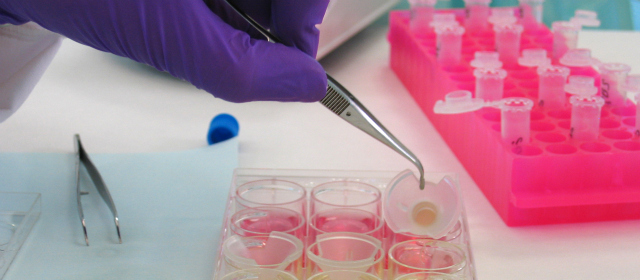
[page_title] 2010 has been another productive year for the Organisation for Economic Co-operation and Development (OECD) test guidelines programme, with multiple EU-pioneered and ECVAM-endorsed 3Rs methods achieving formal international acceptance as OECD guidelines and guidance documents. These include: TG429 (revised) – Local Lymph Node Assay (including the limit dose ‘rLLNA’ protocol) TG439 (new) – In […]
carcinoGENOMICS news

[page_title] The carcinoGENOMICS project board met in Brussels on 27-28 May in combination with a capacity-building meeting. This was an internal meeting, to which regulators from the European Medicines Agency Working Group as well as the Dutch chemical authority RIVM and the Organisation for Economic Co-operation and Development were invited for discussions. The theme of […]
ESNATS update

[page_title] The Embryonic Stem cell-based Novel Alternative Testing Strategies (ESNATS) held its general consortium meeting from 26-28 April at the Joint Research Centre in Ispra, Italy. This meeting had as a main goal to discuss the progress made in the two years since the project’s inception, to discuss and agree on different project-related topics, and […]
ReProTect news

[page_title] The ReProTect project officially ended at the end of 2009 following the final Annual General Meeting in Italy on December 7. In addition to the preparation of a final project report for public dissemination, the report and recommendations of an ECVAM/ReProTect Workshop entitled Implementation of the Embryonic Stem Cell Test has been published in […]
Sens-it-iv update

[page_title] A 4th general assembly of the project was held on 26-28 April in Berlin, during which the work packages had the opportunity to discuss their implementation plan for the next months. In response to feedback from the Scientific Advisory Board, some of the assays under development were (at least temporarily) abandoned due to scientific […]
START-UP Latest news

[page_title] Reports from the three START-UP workshops on refinement, reduction and replacement have been compiled and supplied to the European Commission in April 2010, together with recommendations and road maps. This final document will be used to produce a booklet about the START-UP project for distribution to the general public.
